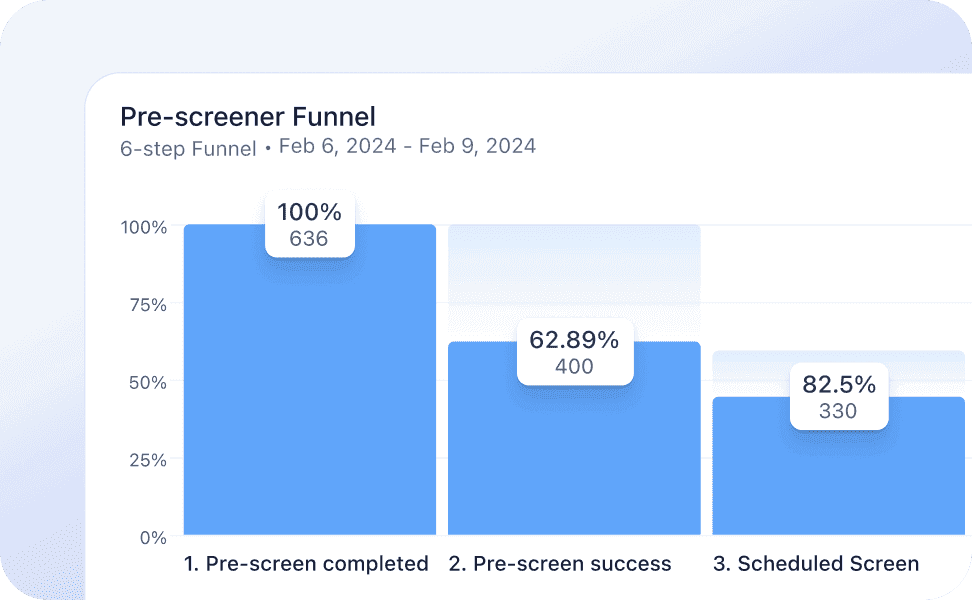
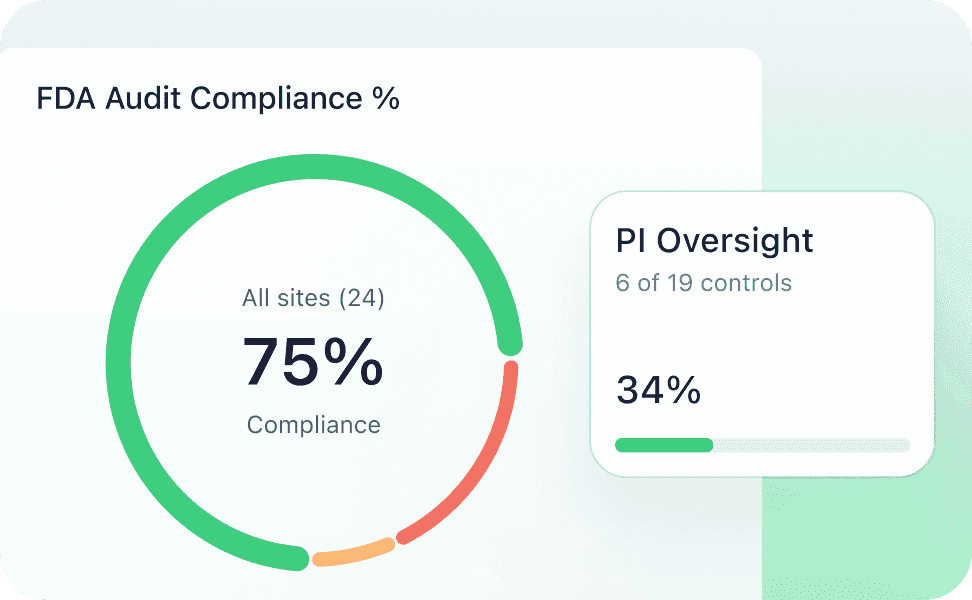
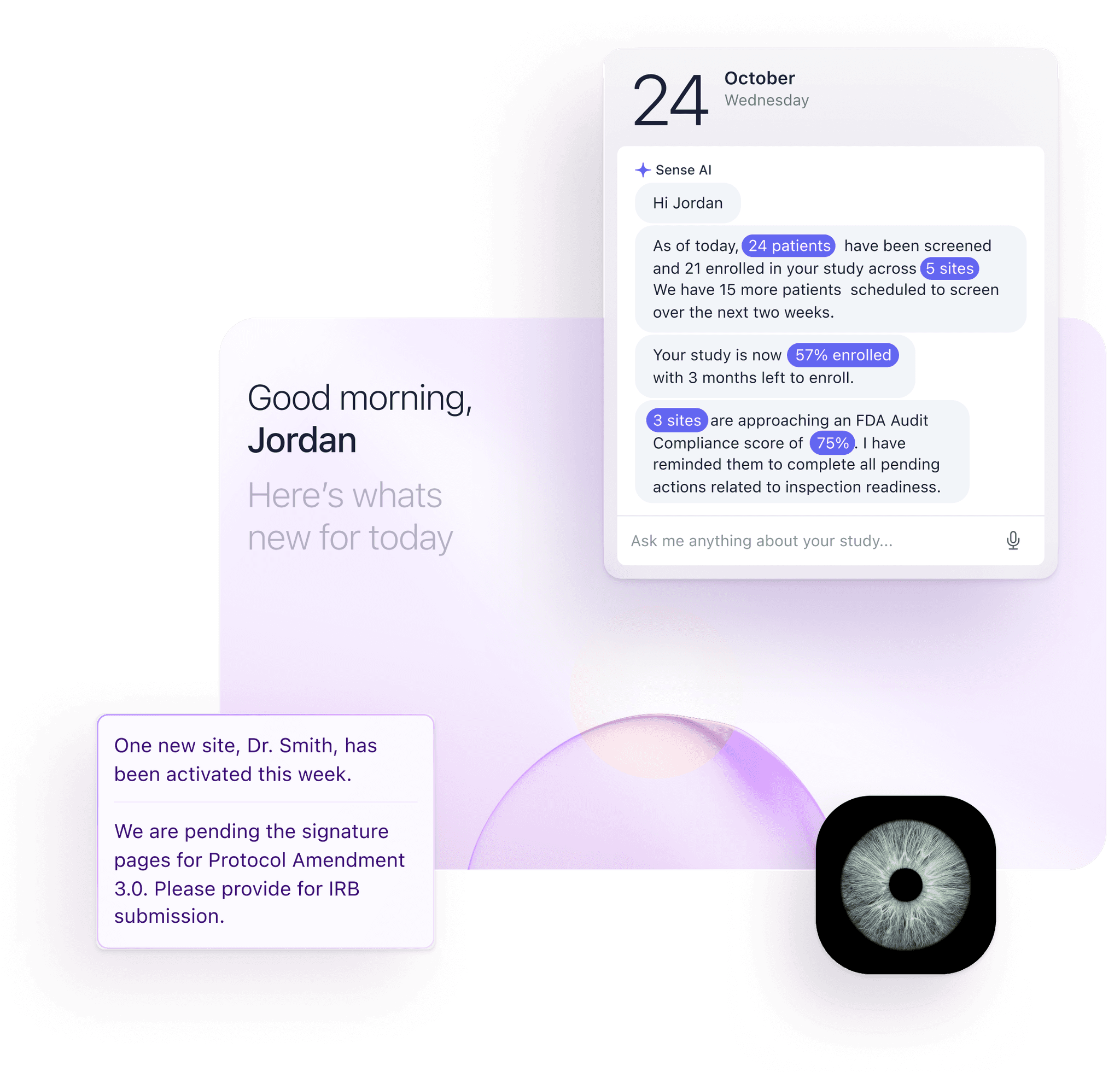
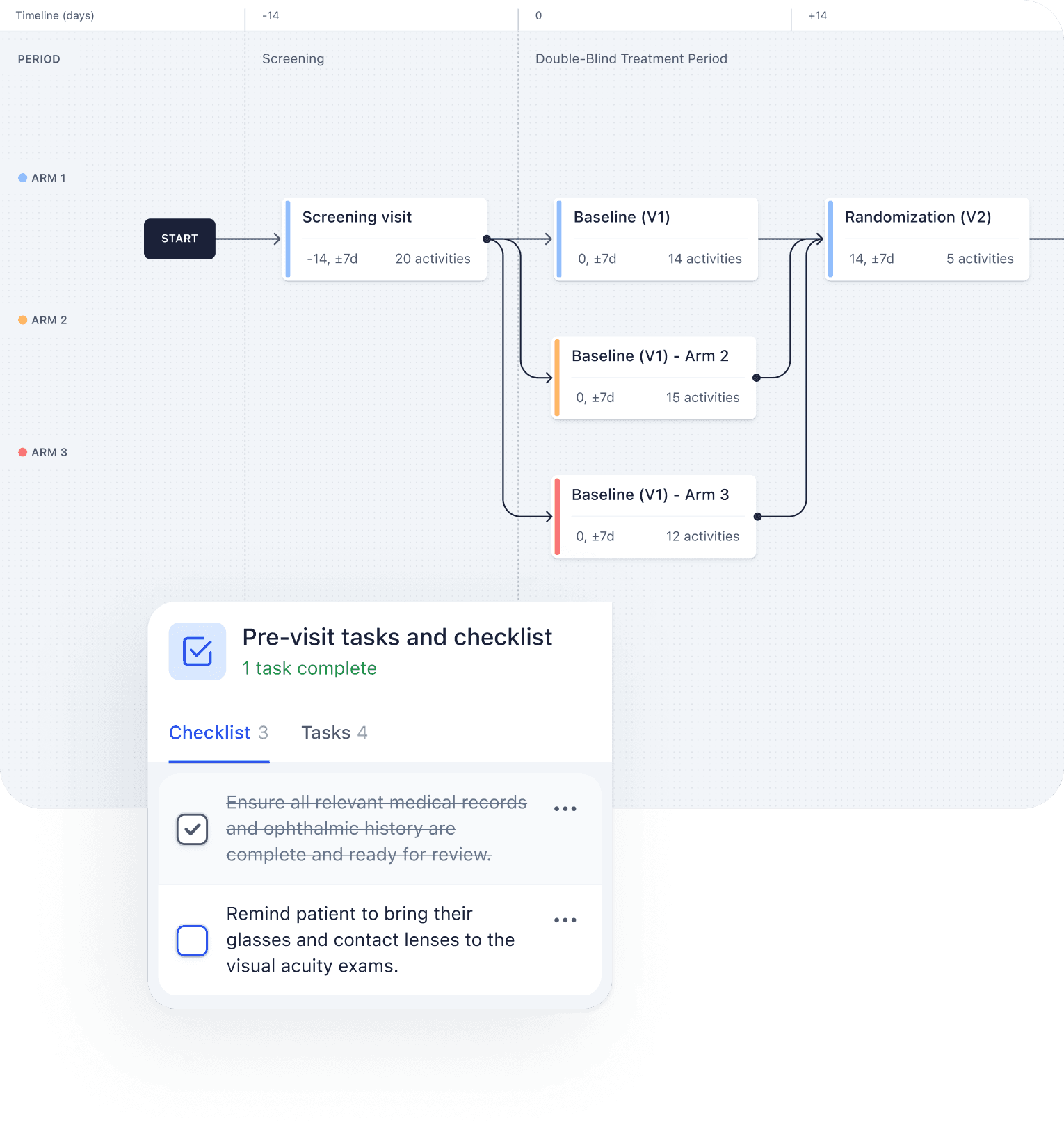
Our unique, automation-first approach, together with our deep ophthalmology expertise, helps you meet your clinical endpoints efficiently. From protocol development all the way to regulatory submission, the highly experienced Mintra Health team ensures that your trials run smoothly and deliver results.
Better data quality and faster timelines
Extensive automation results in demonstrably better data quality and faster timelines.
Predictability in Delivery
Our technology-driven approach leads to more accurate and predictable costs, with minimal deviations.
Consistency and compliance
Guided workflows drive consistency of study execution and improved protocol compliance.
What We Offer
Accelerated Timelines
Using an advanced Workflow AI platform, we can automate 50% of study management, and over 80% of study set-up and close out, substantially reducing time and cost.
Quality Control
Real-time monitoring ensures high-quality data and protocol compliance.
Flexibility
Our team is composed of agile, creative thinkers who tailor each project to your specific requirements.
Mintra Health is led by clinical experts who have extensive experience helping sponsors achieve successful study outcomes. This includes experts in clinical operations, QA, data management, biostatistics and statistical programming, medical monitors and regulatory experts.
Experienced Site Partners
We partner only with the most competent ophthalmic research investigators. Our site partners are extensively pre-vetted and trained on the technology to ensure maximum success.
The Mintra Difference
At Mintra Health, we offer end-to-end clinical trial solutions with an unwavering focus on delivering high-quality results. Our services span the entire clinical trial lifecycle, from start-up to close-out, ensuring seamless operations, patient safety, and data integrity.
Shortened Timelines
Automate tasks typically done manually, shortening study timelines.
Increased Quality Control
Programmed controls allow targeted real-time management of site performance and compliance.
Improved Communication
Provide sponsors with transparent and immediate access to study details.
Reduced Burden on Sites
Alleviate manual efforts at every phase of the trial.